Cancer and Medicinal Mushrooms: Clinical Trials and Practice
Besides tens of thousands of cell culture and animal model experiments, anticancer effects of medicinal mushrooms have been observed in human studies, including clinical trials.
Every drug development includes several clinical trials. Official anticancer drugs from mushrooms are PSK (Krestin; since 1977), Lentinan (1985) and SPG (Sonifilan; 1986) in Japan and PSP (1983) in China. All of them are still used today. Many clinical trials also examined the simultaneous use of these drugs with various chemotherapy and radiotherapy, for many types of cancer.
Clinical studies have shown that the active compounds from medicinal mushrooms work best when combined with surgery, chemotherapy, and radiotherapy. Adding medicinal mushroom drugs greatly improve the outcome and tolerance to invasive treatments. This is now the routine medical approach to cancer in Japan and China.
In the early 1980s, Taguchi et al. tested lentinan, a beta glucan from shiitake, on phase III stomach cancer (advanced or recurrent) in 275 patients. Lentinan was used in combination with cytostatic chemotherapy. The study proved that adding lentinan safely:
- prolongs life
- reduces cancer treatment side effects
- improves the patient’s immune response to cancer.
In another clinical study, Kasamatsu et al. tested PSK, from Trametes versicolor, on phase III cervical cancer. They have found that PSK greatly prolongs life in combination with radiotherapy and makes cancer cells more sensitive to radiation. 5-year survival rates differed drastically: 48% (without PSK) vs. 79% (with PSK).
Published in 1990, a clinical trial by Mitomi et al. on 462 patients showed that PSK, in combination with a cytostatic (type of chemotherapy drug), improves disease-free survival in resected bowel cancer (colorectal cancer; cancer of colon and/or rectum), when compared to using chemotherapy alone.
In China, Q. Y. Yang et al. carried out a study on 485 patients with esophageal, stomach and lung cancer, establishing that PSP:
- alleviates the side effects of standard cancer therapy
- increases recovery and survival rate
- inhibits cancer by activating T-lymphocytes, NK cells and complement C3.
No clinical trials have yet been carried out in the West, mostly due to lack of tradition, higher costs and overly restrictive health authorities.
All clinical trials regarding medicinal mushrooms and cancer only tested single compounds from just three species: Lentinus edodes (shiitake), Trametes versicolor (Turkey tail) and Schizophyllum commune (Split Gill fungus). Ganoderma lucidum (reishi, ling zhi), which is probably the most famous, was not even tested.
Low dosages and short duration is the problem of almost all clinical trials done in the East; the studies that lasted longer showed best results. Even so, the results of clinical studies regarding cancer and medicinal mushrooms are very convincing.
In 2005, J. Sakamoto, S. Morita, K. Oba, T. Matsui, M. Kobayashi, H. Nakazato and Y. Ohashi published the meta-analysis of three randomized clinical trials on the efficacy of PSK use in patients with curatively resected colorectal cancer. This meta-analysis encompasses 1,094 patients, who were followed-up at least 5 years after the surgery and outcomes of the standard chemotherapy were compared with those for chemotherapy plus PSK. While 5-year survival rate of patients with chemotherapy alone was 72.2%, survival rate of patients treated with PSK in addition to chemotherapy was 79.0%. After 5 years, recurrence was found in 34.1% of patients treated with chemotherapy alone and 27.8% in patients with PSK added to standard treatment. These data show that use of immunotherapeutic agent PSK combined with standard oncological therapy significantly improves not only overall 5-year survival rate, but also disease-free survival rate.
K. Oba, S. Teramukai, M. Kobayashi, T. Matsui, Y. Kodera and J. Sakamoto published another meta-analysis of 8 randomized controlled trials on efficacy of PSK treatment of patients with curatively resected gastric cancer in 2006. The 8 clinical trials included 8,009 patients and showed that five-year survival rates in patients treated with PSK alongside standard therapies were increased from 0.9-13.2 % in comparison with chemotherapy alone.
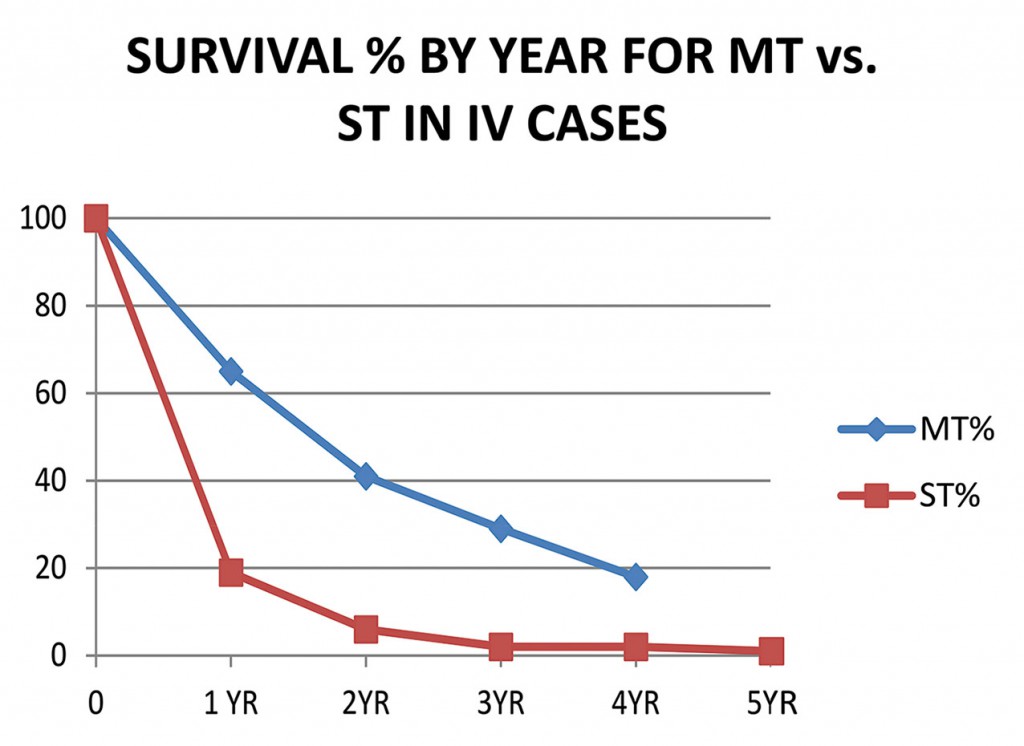
Myko San Cohort Studies
We at Myko San have completed 3 cohort studies: on 65 lung cancer, 51 bowel (colorectal) cancer and 105 breast cancer patients. (also see longer term bowel and breast cancer study) Cohort studies are longer-term observational studies that compare treated group to the control group, which is outside the control of the investigator. The results have been presented at the 4th, 5th and 6th International Medicinal Mushroom Conferences and published in the International Journal of Medicinal Mushrooms.
Myko San studies were single blind: all patients received our supplements, and there was no placebo control. We analyzed the official medical documentation from non-affiliated doctors (in Croatian and other hospitals) and compared the short-term and long-term effects of using our mushroom extracts with the results of standard therapy published in official US cancer registers, as control group. (The US statistics that we used for the control group were significantly better than Croatian, which have been unavailable at the time.)

Patients with lung cancer, bowel (colorectal), and breast cancer using Myko San products showed greatly
- improved survival and health status (esp. in advanced recurrent and metastatic tumor disease)
- lessened side effects of standard cancer therapy
- improved quality of life
compared to patients who only received standard cancer therapy.
In our cohort studies, we:
- applied large doses
- combined multiple active compounds from medicinal mushrooms
- used for a longer period of time (on average 3 months).
It has led to greatly improved outcome and increased survival in lung cancer (small and non-small lung carcinoma), bowel cancer (colorectal cancer), and breast cancer.
Most clinical trials in the East were done in the 1980s and early 1990s. Since then, research has found that larger doses are completely safe and more effective. In our research, we used much larger doses, since antitumor effects are strongly dose-dependent. This relationship is a strong proof of medicinal mushrooms anticancer potential. In our studies, even with very large doses, we observed no significant side effects.
Second, our products are a blend of several medicinal mushroom extracts, combining many active compounds. In our 25-year experience with tens of thousands of cancer patients we have noticed it just works better. Newer research suggested it; our published research has finally proved it. (Durgo et al. Cytotoxicity of Blended vs. Single Medicinal Mushroom Extracts on Human Cancer Cell Lines. International Journal of Medicinal Mushrooms v15.i5, p.435-48, 2013). High quality blends work better because they target more stages of carcinogenesis and tumor growth, meaning they activate more antitumor mechanisms.